Stat EMS™
Not yet available in the U.S. or Canada
 Ambulance and Emergency Blood Testing
Ambulance and Emergency Blood Testing
Stat EMS measures glucose, ketone, lactate, hematocrit, and hemoglobin from a capillary blood drop. Results are available in seconds, depending on the test. By combining single-use biosensor technology with portable electronics, Stat EMS offers simple, fast, accurate testing in the field.
Test Menu
Stat EMS provides an important test menu for immediate patient assessment and faster, more effective emergency treatment. Stat EMS also assists with rapid triage and determining the appropriate transport site for patients with trauma, sepsis, or other specialized needs.
Glucose and Ketone
Abnormal glucose levels are frequently encountered in emergency medicine. Medical conditions that warrant pre-hospital blood glucose testing include diabetes, altered mental state, abnormal vitals, fever, illness, nausea, infection, seizure, acute coronary syndrome, organ injury, trauma, sepsis, septic shock, and burns.1-3Blood ketone testing provides rapid detection or rule-out of diabetic ketoacidosis (DKA), the leading cause of hospitalization and death for children with diabetes.4 The American Diabetes Association and International Society for Pediatric and Adolescent Diabetes recommend blood ketone testing for people with diabetes whenever glucose is above 14 mmol/L and during illness.5,6
Lactate
Elevated blood lactate is a rapid, sensitive indicator of tissue hypoxia, sepsis, septic shock, and hypovolemic shock.7-9In pre-hospital medicine, lactate testing provides:
- Early, more sensitive detection of sepsis and septic shock than vital signs alone8
- Identification of patients with sepsis who might benefit from early goal-directed therapy and advanced activation of medical staff at the transport site10,11
- Evaluation of trauma, critical illness, hemorrhage, acute coronary syndrome, acute respiratory failure, and chest abdominal pain11-13
Hemoglobin and Hematocrit
Stat EMS provides accurate, measured results for both hemoglobin and hematocrit for pre-hospital assessment of blood oxygen carrying capacity, and for:
- Rapid identification of chronic and acute anemia14
- Evaluation of internal or external hemorrhage
- Assessment of the need for blood products14,15
- Estimation of blood loss15
 Carrying and charging case
Carrying and charging case
A compact, rugged carrying case contains
all testing components, including meters,
biosensors, controls, and lancets. It also serves as the battery charging station
for the meters.
The case is 41 cm x 36 cm x 15 cm
(16 in x 14 in x 6 in).
Fingerstick Sampling
Using capillary samples as small as 0.6 µL, Stat EMS provides fast and easy testing for high-stress medical situations. Capillary sampling is as easy as glucose self-testing performed by patients with diabetes. It eliminates the time and costs of a venipuncture, including the tourniquet, needle, vacutainer, and transfer pipette—as well as finding a suitable vein.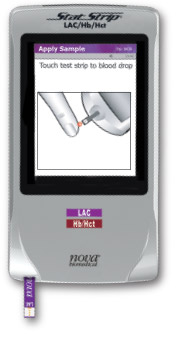 Simple to Use
Simple to Use
Stat EMS meters do not require coding
or calibration before use.
Testing steps:
- Insert biosensor into meter.
- Lance finger to obtain blood drop.
- Touch biosensor to blood drop.
- Read results.
Results in Seconds
With fast, simple testing steps and ready-to-use biosensors, Stat EMS provides results in
6 to 40 seconds.
Data Storage and Connectivity
The meters store up to 1,000 patient results and offer wireless and Bluetooth connectivity to any enabled device through industry standard POCT1-A2, HL7, and ASTM formats. Stat EMS also offers hardwired connectivity to other electronic data systems.Portable for Field Use
Compact, light, and rugged, the Stat EMS carrying case is designed for mobile testing in the field. The case weighs only three pounds when holding all testing components, including meters, biosensors, controls, and lancets. It's water resistant and designed to protect all components if dropped. The case and meters can be cleaned and disinfected with wipes containing bleach.
Nova Biomedical – A World Leader in Critical Care and Point-of-Care Testing
Nova Biomedical has been developing and building advanced technology whole blood analysers for hospitals worldwide for over 40 years. Nova has developed over 20 whole blood biosensors, and more than 200 peer-reviewed studies have validated the accuracy of our biosensor measurement technology. Because of its superior accuracy Nova’s glucose biosensor is the only cleared by the U.S. Food and Drug Administration for testing critically ill patients. Thousands of hospitals around the globe trust Nova’s technology to measure blood from critically ill patients—the same technology Nova is bringing to ambulance, pre-hospital and emergency medicine.
Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800
Stat EMS Specifications
Meters
Glucose and Ketone meter Lactate and Hb/Hct MeterWeight: 220 g (0.49 lb)
Size: 147 mm x 79 mm x 30 mm (5.8 in x 3.1 in x 1.18 in)
Data Storage:
Patient Tests: 1,000
QC Tests:200
Battery Information:
Type: 3.7V Li Polymer Battery
Features: Rechargeable
Life: Minimum 600 tests
Operating Ranges:
Temperature:1 ̊C–40 ̊C (34 ̊F–104 ̊F)
Altitude: Up to 4,572 meters
Humidity: 10%–90% relative humidity
Carrying Case and Charging System
Weight:
1.6 kg (3 lb)
Size:
41 cm x 36 cm x 15 cm (16 in x 14 in x 6 in)
Certifications and Compliance:
Nova Biomedical is certified to FDA Quality System Regulations and EN ISO 13485:2016
Complies to IVDD Tested according to: EN 61010-1:2010, EN 61010-2-101:2015, EN 60825-1/A1:2014
Nova Biomedical Patent Numbers:
CA2846887A1 EP2568281A1 US8603309 JP5812957 US9535053B1 US8603309B2 US9638686B1
Nova Biomedical® is a registered trademark of Nova Biomedical Corporation.
Biosensor Specifications
StatStrip Glucose Biosensor
Only glucose biosensor that's FDA cleared for monitoring dysglycemia in critically ill patients

Test Measured: Blood Glucose
Test Time: 6 seconds
Test Strip Volume: 1.2 µL
Test Methodology: Electrochemistry
Sample Types:
Whole Blood: Arterial, Capillary, Venous
Measurement Range:
Glucose: 0.6–33.3 mmol/L (10–600 mg/dL)
Operating Ranges:
Temperature: 1˚C–40˚C (34˚F–104˚F))
Altitude: Up to 4,572 m (15,000 ft)
Humidity: 10%–90% relative humidity
Reagents and Strips:
Test Strips: 2 vials of 50
QC: 3 levels (sold separately)
Linearity: 5 levels available
Test Strip Use Life: 24 months from
date of manufacture
StatStrip Lactate Biosensor
Best biomarker for detecting and guiding therapy for severe sepsis, septic shock,
and trauma

Test Measured: Blood Lactate
Test Time: 13 seconds
Test Strip Volume: 0.6 µL
Test Methodology: Electrochemistry
Sample Types:
Whole Blood: Arterial, Capillary, Venous
Measurement Range:
Lactate: 0.3–20 mmol/L
Operating Ranges:
Temperature: 1˚C–40˚C (34˚F–104˚F))
Altitude: Up to 4,572 m (15,000 ft)
Humidity: 10%–90% relative humidity
Reagents and Strips:
Test Strips: 2 vials of 25
QC: 3 levels (sold separately)
Linearity: 5 levels available
Test Strip Use Life: 24 months from
date of manufacture
StatStrip Ketone Biosensor
Best biomarker for detecting and guiding therapy for ketosis and DKA

Test Measured: Blood Ketone
Test Time: 10 seconds
Test Strip Volume: 0.8 µL
Test Methodology: Electrochemistry
Sample Types:
Whole Blood: Arterial, Capillary, Venous
Measurement Range:
Ketone: 0.1–7.0 mmol/L
Operating Ranges:
Temperature: 1˚C–40˚C (34˚F–104˚F))
Altitude: Up to 4,572 m (15,000 ft)
Humidity: 10%–90% relative humidity
Reagents and Strips:
Test Strips: 2 vials of 25
QC: 3 levels (sold separately)
Linearity: 5 levels available
Test Strip Use Life: 24 months from
date of manufacture
StatStrip Hb/Hct Biosensor
Rapid diagnosis of blood loss and anemia

Test Measured: Hemoglobin and Hematocrit
Test Time: 40 seconds
Test Strip Volume: 1.6 µL
Test Methodology: Electrochemistry
Sample Types:
Whole Blood: Capillary, Venous
Measurement Range:
Hemoglobin: 6.5–22 g/dL
Hematocrit: 20%–65%
Operating Ranges:
Temperature: 1˚C–40˚C (34˚F–104˚F))
Altitude: Up to 4,572 m (15,000 ft)
Humidity: 10%–90% relative humidity
Reagents and Strips:
Test Strips: 2 vials of 25
QC: 3 levels (sold separately)
Linearity: 3 levels available
Test Strip Use Life: 24 months from
date of manufacture
References
1. Navarro K. Blood glucose test for altered mental status. EMS1. 31 May 2013. https://www.ems1.com/ems-products/Ambulance-Disposable-Supplies/articles/1454354
2. Khoujah D et al. Status epilepticus. What’s new? Emerg Med Clin N Am 2016;34:759-776.
3. Solnica B. [Diagnostic aspects and analytical problems of glycemia monitoring in intensive care unit patients.] Przegl Lek 2006;63(9):792-796.
4. Bismuth E et al. Can we prevent diabetic ketoacidosis in children? Pediatr Diabetes 2007;8(Suppl. 6):24-33.
5. American Diabetes Association. Tests of glycemia in diabetes. Diabetes Care 2004;27(Suppl. 1):S92.
6. Rewers MJ et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014;15(Suppl. 20):102-114.
7. Bakker J et al. [Serum lactate level as a indicator of tissue hypoxia in severely ill patients]. Ned Tijdschr Geneeskd 2000;144(16):737-741.
8. Jansen TC. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: A pilot study. Crit Care 2008;12:R160.
9. Andersen LW et al. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc 2013;88(10):1127-1140.
10. Guerra WF et al. Early detection and treatment of patient with severe sepsis by prehospital personnel. J Emerg Med 2013;44(6):1116-1125.
11. St. John AE et al. Prehospital lactate predicts need for resuscitative care in non-hypotensive trauma patients. West J Emerg Med 2018;19(2)224-231.
12. Vincent JL et al. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit Care 2016;20(1):257.
13. Soremekun OA et al. Utility of point-of-care testing in ED triage. Am J Emerg Med 2013;31(2):291-296.
14. Vieth J et al. Anemia. Emerg Med Clin N Am 2014;32:613-628.
15. Figueiredo S et al. How useful are hemoglobin concentration and its variations to predict significant hemorrhage in the early phase of trauma. A multicentric cohort study. Ann Intensive Care 2018;8:76.